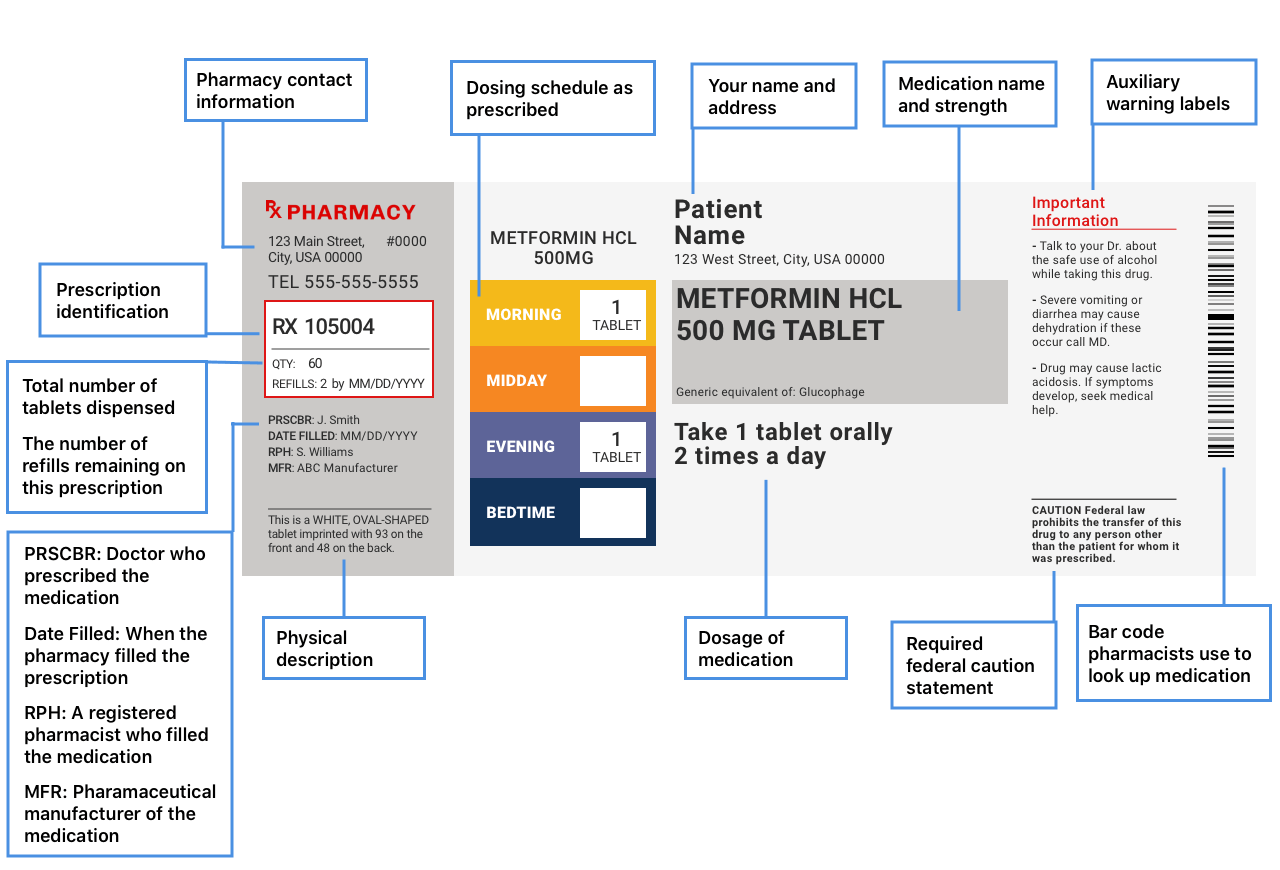
Federal Prescription Label Requirements . prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100 (d) must meet the following general requirements: this web page contains the federal regulations for prescription drug labeling, including the required information.
from www.drugwatch.com
this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100 (d) must meet the following general requirements: prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and.
How to Read OvertheCounter and Prescription Drug Labels
Federal Prescription Label Requirements learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. this web page contains the federal regulations for prescription drug labeling, including the required information. prescription drug labeling described in § 201.100 (d) must meet the following general requirements:
From hpr.com
Prescription Label 03 Hand Prop Room Federal Prescription Label Requirements this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the types, features, and approval process of labeling for prescription. Federal Prescription Label Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Federal Prescription Label Requirements learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. this web page contains the federal regulations for prescription drug labeling, including the required information. prescription drug labeling described in § 201.100. Federal Prescription Label Requirements.
From kiwikellz.blogspot.com
28 Federal Prescription Label Requirements Labels Ideas For You Federal Prescription Label Requirements learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. this web page contains the federal regulations for prescription. Federal Prescription Label Requirements.
From www.languageconnections.com
Do You Understand Your Prescription? Language Connections Blog Federal Prescription Label Requirements learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100. Federal Prescription Label Requirements.
From kiwikellz.blogspot.com
28 Federal Prescription Label Requirements Labels Ideas For You Federal Prescription Label Requirements prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. this web page contains the federal regulations for prescription. Federal Prescription Label Requirements.
From www.researchgate.net
2Prescription label (actual size). Download Scientific Diagram Federal Prescription Label Requirements prescription drug labeling described in § 201.100 (d) must meet the following general requirements: learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the fda's labeling requirements and guidance for medical devices,. Federal Prescription Label Requirements.
From brennad-images.blogspot.com
Printable Prescription Warning Labels / Ers Solutions Pharmacy Dispensing Labels Choose from Federal Prescription Label Requirements prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. this web page contains the federal regulations for prescription drug labeling, including the required information. prescription drug labeling described in § 201.100 (d) must meet the following. Federal Prescription Label Requirements.
From dandelionsandthings.blogspot.com
34 Nys Prescription Label Requirements Label Design Ideas 2020 Federal Prescription Label Requirements learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100(d) must meet the following general requirements: this web page contains the federal regulations for prescription. Federal Prescription Label Requirements.
From kiwikellz.blogspot.com
28 Federal Prescription Label Requirements Labels Ideas For You Federal Prescription Label Requirements learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. this web page contains the federal regulations for prescription drug labeling, including the required information. prescription drug labeling described in § 201.100(d) must meet the following general requirements: prescription drug labeling described in § 201.100 (d) must meet the following. Federal Prescription Label Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Federal Prescription Label Requirements learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. prescription drug labeling described in § 201.100(d) must meet the following general requirements: this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical. Federal Prescription Label Requirements.
From animalia-life.club
Fda Drug Labeling Requirements Federal Prescription Label Requirements this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. prescription drug labeling described in § 201.100(d). Federal Prescription Label Requirements.
From hub.arkansasbluecross.com
Deciphering Your Prescription Medication Label Blueprint Federal Prescription Label Requirements prescription drug labeling described in § 201.100 (d) must meet the following general requirements: this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. learn about the types, features, and approval process of labeling for. Federal Prescription Label Requirements.
From kiwikellz.blogspot.com
28 Federal Prescription Label Requirements Labels Ideas For You Federal Prescription Label Requirements prescription drug labeling described in § 201.100 (d) must meet the following general requirements: learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. prescription drug labeling described in § 201.100(d) must. Federal Prescription Label Requirements.
From ar.inspiredpencil.com
Fda Drug Labeling Requirements Federal Prescription Label Requirements learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100(d) must meet the following general requirements: this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the types, features, and approval process of labeling for prescription. Federal Prescription Label Requirements.
From my.clevelandclinic.org
How To Read A Prescription Label A Guide Cleveland Clinic Federal Prescription Label Requirements learn about the types, features, and approval process of labeling for prescription medicines, including prescribing information, carton and. prescription drug labeling described in § 201.100(d) must meet the following general requirements: this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical. Federal Prescription Label Requirements.
From rxoutreach.org
Education Understanding Prescription Medication Labels Rx Outreach Federal Prescription Label Requirements prescription drug labeling described in § 201.100(d) must meet the following general requirements: learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100 (d) must meet the following general requirements: learn about the types, features, and approval process of labeling for prescription medicines,. Federal Prescription Label Requirements.
From www.drugwatch.com
How to Read OvertheCounter and Prescription Drug Labels Federal Prescription Label Requirements this web page contains the federal regulations for prescription drug labeling, including the required information. learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. prescription drug labeling described in § 201.100(d) must meet the following general requirements: prescription drug labeling described in § 201.100 (d) must meet the following. Federal Prescription Label Requirements.
From exoisyjew.blob.core.windows.net
Medicine Labels Examples at John Cruz blog Federal Prescription Label Requirements prescription drug labeling described in § 201.100 (d) must meet the following general requirements: learn about the fda's labeling requirements and guidance for medical devices, including advertising, symbols, unique device. this web page contains the federal regulations for prescription drug labeling, including the required information. prescription drug labeling described in § 201.100(d) must meet the following. Federal Prescription Label Requirements.